This post is also available in: 简体中文 繁體中文
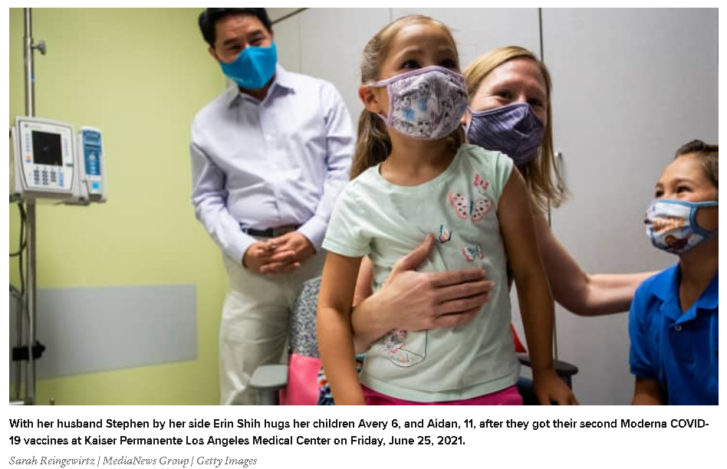
Moderna said Monday a smaller dosage of its Covid-19 vaccine is safe and generates a strong immune response in a study of children ages 6 to 11.
Two 50-microgram doses of the vaccine, half the dosage given to adults, produced antibody levels that were 1.5 times higher than those seen in young adults, the company said in a press release, citing early data from a phase 2/3 trial.
The shots were also generally well-tolerated in young kids, according to the company, with the most common side effects being fatigue, headache, fever, and injection-site pain. The vaccine was tested on more than 4,700 children.
Moderna said it plans to submit the data to the Food and Drug Administration, European Medicines Agency and other health regulators in the “near term.”
“We look forward to filing with regulators globally and remain committed to doing our part to help end the COVID-19 pandemic with a vaccine for adults and children of all ages,” Moderna CEO Stephane Bancel said in a statement.
The new data comes a day before a key FDA advisory committee meets to discuss whether to recommend Pfizer and BioNTech’s vaccine for kids ages 5 to 11. The FDA could authorize the shots within days of the meeting, and the Centers for Disease Control and Prevention could authorize it as early as next week.
FDA staff said late Friday that Pfizer’s vaccine appears highly effective at preventing symptomatic infections in elementary school-age children.
Many parents say they are anxious to get their children vaccinated as kids started the new school year with the delta variant still surging across America. The number of new Covid cases in kids remains exceptionally high, with more than 1.1 million child cases added over the past six weeks, according to the American Academy of Pediatrics.
Article From: CNBC
Author: Berkeley Lovelace Jr.

