Variants of SARS-CoV-2 arise through errors in the virus’s replication process. But some mistakes give the virus a deadly advantage.
Salim Abdool Karim had never seen his colleague so quiet.
Tulio de Oliveira, who normally buzzes with enthusiasm, skipped all his usual banter as he walked into Abdool Karim’s office. The bioinformatician sat down, plugged his laptop into a projector, and up popped a series of diagrams representing sequences of the SARS-CoV-2 virus onto the large screen.
“Twenty-three mutations,” de Oliveira told his colleague. “We have 23 mutations.”
For the previous seven months, Abdool Karim, an epidemiologist and head of a committee advising the South African government on its pandemic response, and de Oliveira had met regularly to review the country’s data on SARS-CoV-2, the virus that causes the disease COVID-19. The pair, who work at the Centre for the AIDS Programme of Research at South Africa’s Nelson Mandela Medical School at the University of KwaZulu-Natal, were looking for changes in the genomic sequence of the virus circulating in South Africa. Every month, the researchers picked up one, maybe two mutations, but by fall, they’d seen nothing of consequence.
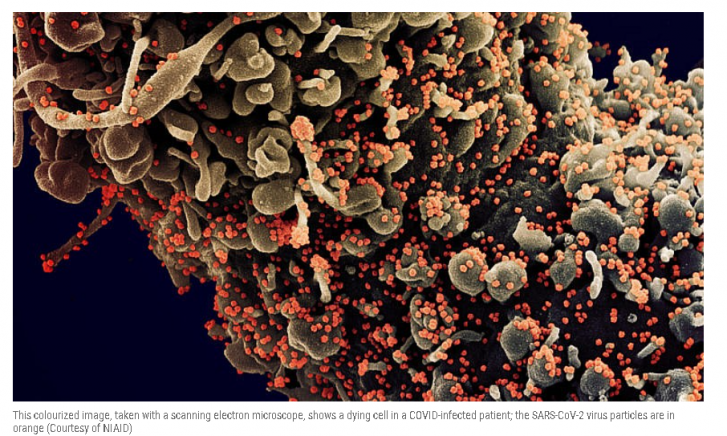
The whole thing, frankly, had become a little boring. Unlike HIV, which mutates at lightning speed, SARS-CoV-2 plodded along rather reliably. Abdool Karim, a renowned researcher in HIV who is often called South Africa’s Anthony Fauci, began to wonder if they were wasting their time. “I started to think Tulio was just coming to my office because he liked the coffee,” he says.
But on this day in late November, everything changed. Not only did the two scientists see 23 mutations in sequences, three of the mutations were located in the receptor-binding domain of the virus—part of the spike protein on the virus’s exterior that unlocks entry into a human cell.
“Where did this come from?” Abdool Karim asked his colleague. “We haven’t seen a single mutation in that area [of the virus]. Not one. You’re telling me there’s three in one month?” No one anywhere had reported anything like this, despite a global network of scientists who’d been tracking SARS-CoV-2 for the better part of a year.
Abdool Karim and de Oliveira set to work, trying to figure out what these mutations meant before they made their finding public. They knew only that this variant of SARS-CoV-2 was linked to an outbreak of COVID-19 on the Eastern Cape of South Africa. One clue appeared in a paper published in the journal Cell, in which scientists changed amino acids on the spike protein to predict what might happen. As they read the paper, the South Africans’ anxiety grew. The model suggested that at least two of the mutations they were seeing on this variant of SARS-CoV-2 would allow the virus to latch more tightly to the angiotensin-converting enzyme 2 (ACE2) receptor on human cells where the virus finds its way in. “Suddenly, there’s a strong attachment [between the virus and the cell],” Abdool Karim explains.
They worried these mutations might help the virus escape immunity. And their concern was setting in just as people around the world were celebrating vaccines. On Dec. 2, 2020, the United Kingdom approved the Pfizer-BioNTech vaccine, the first country in the world to do so.
The South African scientists reached out to colleagues in the U.K., home to the world’s largest genomic surveillance program, the COVID-19 Genomics U.K. Consortium. There, more than 14,000 km away from Abdool Karim’s office in Durban, researchers pored through their own data and picked up a variant that had been circulating for weeks, driving a rise in COVID-19 in southeast England. Like the variant in South Africa, it had a mutation on the spike protein at the 501st amino acid—asparagine had changed to a tyrosine amino acid, known as an N to Y mutation.

The British announced their variant to the world first, calling it the 501Y.V1 variant—the “Y” for tyrosine and “V1” for version one. It’s also known as B.1.1.7 in recognition of its family—or phylogenetic—tree. Neither is a particularly catchy name, so the moniker “U.K. variant” stuck, despite the World Health Organization (WHO)’s ongoing caution against naming diseases or versions of them after geographic locations. (On May 31, 2021, the WHO introduced a new naming protocol for variants, using Greek letters. B.1.1.7, for example, is now known as Alpha.) On Dec. 14, Nick Loman, professor of microbial genomics and bioinformatics at the University of Birmingham, told a scientific briefing that the B.1.1.7 variant first popped up in Kent in late September, but within three months it was responsible for 20 per cent of viruses sequenced in Norfolk, a picturesque county on England’s east coast.
A few days later, Abdool Karim and de Oliveira’s team announced their variant to the world through a live broadcast on South African television, calling it 501Y.V2 for version two. It’s also known as B.1.351.
Before long, there was another: in January, researchers detected a new variant of concern in four travellers from Brazil who were tested with routine screening at Haneda Airport in Tokyo. This became 501Y.V3, the third version of SARS-CoV-2 with a mutation at 501. It’s also known as the P.1 variant—the “P” marking a new branch in the phylogenetic tree, having grown from a lineage first detected in March 2020. P.1 arose in Manaus, a city in Brazil where as many as two-thirds of residents were estimated to have had COVID-19 in an earlier wave. Experts had believed there was a high degree of immunity among the population. But by January, case numbers were in resurgence—an indication that this new variant was dodging the immunity generated by past infections.
Today, a global hunt for variants is under way. On May 10, the WHO classified B.1.617.2, a variant circulating in India, as a variant of concern—bringing the total to four. Scientists have identified multiple variants of interest emerging around the world, notably in the United States, Rwanda and the Philippines, though these are not yet at the status of concern.
Plenty of questions are swirling about what these variants mean. But this much is clear: they have changed the endgame of the pandemic.
***
A virus exists to do one job: replicate itself. But it can’t accomplish that alone. Instead, a virus invades the cell of a host—that’s us, but might, depending on the virus, also be dogs, cats, bats or minks—and seizes control of the machinery within the host cell to make hundreds or thousands of copies of itself. A protein called a polymerase in the host cell reads the letters of the viral genetic material, representing the chemical bases of adenine, guanine, cytosine and thymine (or uracil in an RNA virus), and the polymerase uses the code as a template to unwittingly make copies of the virus. But the polymerase sometimes makes an error. “Instead of having a T, they put an A, and then you have a mistake in the code,” says Nathalie Grandvaux, professor of biochemistry and molecular medicine at the Université de Montréal.
The process is akin to you taking a page from a book and typing the words out into a Microsoft Word document. Then you’d type another, based on the most recent version, and another, carrying on until you’re out of energy to keep going. You are going to make typos. The same thing happens with viruses. Most RNA viruses, like HIV and influenza, lack any kind of spell check function as they transcribe. They make little errors constantly as they replicate. This is, in part, why the world has not yet developed a successful HIV vaccine that provides long-lasting immunity—the virus changes so quickly that, by the time a person develops antibodies, the antibodies are no match for the circulating virus.
Unlike other RNA viruses, coronaviruses possess a proofreading function, an enzyme known as Nsp14. Nsp14 corrects most mistakes in the transcription, but not all. “It’s still a little bit sloppy,” says Jason Kindrachuk, Canada Research Chair in molecular pathogenesis of emerging and re-emerging viruses at the University of Manitoba. “Coronaviruses mutate to a lesser degree than what we see with things like influenza. But they still mutate.”
Most mistakes don’t matter. Many disappear because they’re not favourable for the virus.
But every so often, a mutation pops up that gives a virus an advantage.
***
In the spring of 2020, doctors in Boston were puzzling over a patient. A 45-year-old man had been admitted to Brigham and Women’s Hospital with COVID-19. He was treated with steroids and went home five days later. But over the next 4½ months, the man came back five more times, requiring hospitalization with symptoms related to COVID-19. Again and again, he tested positive for the coronavirus, even though he quarantined at home between admissions. He’d get sick, receive treatment and improve. Twice, after receiving the drug remdesivir, he tested negative for COVID-19, but only briefly. Every time, the virus roared back.
The patient, who has never been identified, also had a severe autoimmune disease called catastrophic antiphospholipid syndrome. He took medications to suppress his immune system and keep his symptoms in check. His doctors believe that the drugs the man needed to suppress his immune system also left him unable to fight off the coronavirus infection.
Manish Choudhary, a research fellow in medicine at Harvard Medical School, was part of the team called in to assist on the man’s case. Choudhary and colleagues extracted virus samples from the patient and sequenced its genome. They repeated the tests as the man grew sicker. When Choudhary saw the results, he couldn’t believe his eyes. “We thought, ‘Is this real?’ ” he says. The sequences were changing constantly, picking up more than 20 mutations over several months. What’s more, the sequences didn’t match anything in the state’s database—suggesting the changes were happening inside the man’s body and were not reinfections from other people. “You don’t [typically] see these things happening in real time,” says Choudhary, who is an expert in viral evolution during outbreaks. “[But] this is one case where we’re seeing viral evolution happening in front of our eyes.”
In this case, the virus outwitted treatments. After being admitted to the intensive care unit, the man died from respiratory failure and shock, 154 days after his first visit to the hospital.
In November 2020, Choudhary and colleagues published their findings in the New England Journal of Medicine, just a month before Abdool Karim and his colleagues in the U.K. announced the variants circulating in South Africa and England. There was a pattern in these seemingly unrelated cases. The mutations popping up in geographically distinct areas around the world—the Eastern Cape of South Africa, Kent in the United Kingdom and an immunocompromised patient in Boston—were remarkably similar. All had the 501Y mutation. Both the variant reported in South Africa and the American patient also had a mutation called E484K, which is now also present in offshoots of the B.1.1.7 variant. Ordinarily, an amino acid on this position of the SARS-CoV-2 virus carries a negative charge. But the mutation switched the amino acid on the virus to a positive ion. With this change, the cell and the virus are now, theoretically, drawn together like magnets. This mutation may also help the virus escape immunity by limiting the ability of neutralizing antibodies to bind to it and stop the early stage of infection.
“These mutations are what distinguish variants of concern. They give the virus certain properties that cause a lot of consternation and make this a virus we worry much more about,” says Abdool Karim.
The four variants of concern are more transmissible than the original SARS-CoV-2—to varying degrees. The Centers for Disease Control and Prevention estimates that B.1.1.7 is around 50 per cent more contagious and B.1.351, 20 per cent. Because these variants spread faster, they quickly become dominant, wiping out other versions of the virus. The jury is still out on the question of whether these variants also cause more severe disease. Little is known about the B.1.617.2 variant circulating in India, where tragedy has unfolded faster than information can be gleaned about its mutations. Studies of the B.1.1.7 variant are mixed: some suggest it’s more severe, others not. In South Africa, the B.1.351 variant caused more deaths from COVID-19 in January 2021 as cases spiked. But Abdool Karim believes many people died because cases surged and hospitals became overwhelmed. When the country brought in restrictions in January, cases declined, as did the mortality rate. “The excess mortality [in South Africa] disappeared,” he says.

There is even less certainty about whether variants of concern can escape natural immunity. Some do, unquestionably. In Brazil and South Africa, people who were infected in the first wave were reinfected in the second as the variants took hold. “It’s a very sober reminder that, barely six months ago, we had groups of scientists who claimed that the real solution to this pandemic is to just let it spread and to let natural infection save us from this,” says Abdool Karim, referring to the argument put forward by supporters of the Great Barrington Declaration last fall. “Now, we know that that’s completely flawed.”
After Choudhary and colleagues published their report of the Boston patient, similar stories popped up around the world. In one, published in Nature, a man in his 70s who’d received chemotherapy for treatment of lymphoma arrived at his local hospital in the U.K. He tested positive for COVID-19, and did so repeatedly over the next three months, despite receiving treatments of remdesivir and convalescent plasma. Samples showed the virus evolved even more rapidly after plasma therapy, producing mutations in the virus’s genetic code that helped it evade the immune system.
These cases have led many scientists to believe that the variants of concern emerged in immunocompromised patients like the man in Boston and the patient in the U.K.—people who were infected with the coronavirus but unable to mount a robust immune response.
In any viral infection, a tug of war takes place between the body’s immune system and the virus’s ability to persist, Choudhary explains. When the immune system malfunctions, the body can’t efficiently eliminate the virus. The virus gains time to replicate and morph into variations beyond the grasp of the immune system. Choudhary says his patient is an example of this. “It was kind of a breeding ground for the virus to undergo changes and this is where you see most of these [mutations] coming in,” he says. “In a way, the virus found a perfect condition to evade the immune system.”
***
In January 2020, Chinese researchers posted the SARS-CoV-2 genome for the world and kicked off a new era in genomic surveillance—one in which scientists can watch a virus move and evolve in real time. They can perform testing out in the field anywhere in the world using equipment that costs around $5,000 and is the size of an iPhone, and then upload the sequences onto a database using a simple USB drive. “This has completely changed our ability to combat infectious diseases,” says Kindrachuk. In comparison, it took more than 80 years to learn the full genome sequences of the 1918-19 influenza pandemic.
As you’ve been reading this, scientists around the world have been uploading new genomic sequences from SARS-CoV-2 onto national and international databases. Others are picking through the data, looking for patterns or changes. Any kind of mutation serves as a bread crumb for the high-tech detectives tracking the virus across the globe. It’s this kind of genomic surveillance that can identify variants and follow their movements.

At the centre of these efforts is a large international repository called the Global Initiative on Sharing Avian Influenza Data (GISAID), first set up in 2008 to help scientists share data on influenza. GISAID is unique in that users must credit scientists whose data they use, making it attractive to researchers. To date, GISAID has collected more than 1.6 million genome sequences of SARS-CoV-2—more than any other pathogen in history. It was through GISAID that scientists learned that 40 per cent of genomes sequenced in Manaus, Brazil, in December were of the P.1 variant—a red flag that alerted public health researchers to the variant’s power. And it was through GISAID that Emma Hodcroft, a virologist at the University of Bern in Switzerland, identified B.1.177, which arose in Spain in the spring of 2020. After borders opened to Europeans that summer, Hodcroft watched the variant hitchhike around the continent with summer holidaymakers, bounding through Switzerland, Ireland and the U.K. by September. According to an analysis by Public Health Ontario and the Canadian COVID-19 Genomics Network (CanCOGeN) published in the Journal of Clinical Virology Plus, B.1.177 arrived in Canada in late September 2020 with a traveller returning from Europe.
Jeffrey Joy, an assistant professor of medicine and an expert in evolutionary genetics of infectious diseases at the University of British Columbia, uses GISAID data to study how SARS-CoV-2 sequences enter and move around Canada. In a study published as a preprint in late April, he and his colleagues found that most new strains of SARS-CoV-2 were imported into Canada via the United States, with a swell in early March 2020. When Canada imposed travel restrictions later that month, the rate of international seeding fell, but did not end. New sublineages continued to squeak into Canada well after April 2020. Once in the country, these strains of SARS-CoV-2 found a rich environment for transmission and spread throughout the provinces.
***
From the virus’s perspective, year two of the pandemic is going swimmingly. After all, its job is to replicate. In the last two weeks of April 2021, more global COVID-19 cases were reported than in the first six months of the pandemic. The virus’s ability to mutate into more contagious versions of itself has fuelled hot spots for COVID-19 around the world, Canada among them.
This chapter of the pandemic is often presented as a final furious contest between variants and vaccines. But scientists who work in this field say the reality is far more complex. The finish line isn’t in sight. The more people the virus infects, the more routes it has to replicate; the more routes it has to replicate, the greater the chance it will undergo changes.
“It’s really a race to keep cases low so you can vaccinate your population before there are too many cases,” says Jesse Shapiro, associate professor in the department of microbiology and immunology at McGill. “So, yes, we have to vaccinate people as quickly as possible, but we also have to keep the variants down by other means—distancing and reducing contacts and reducing travel and all those other things.”
Shapiro says a “worst-case scenario” for variants is to have a large proportion of the population unvaccinated in an environment of high transmission. This will put pressure on the virus, he says. “If cases are really high, even vaccinated people are being exposed to viruses. The viruses are being tested.” Joy agrees: “These variants are going to continue to arise until we’re able to suppress the transmission of the virus to the level where viral evolution becomes more difficult.”
Some scientists are concerned that Canada’s strategy of delaying the second dose of vaccine could increase the risk of variants arising in elderly or immunocompromised patients. They want second doses given to at-risk patients at a faster schedule. “The fact that we give the second dose four months later, for me, that’s taking a chance of having mutants emerging, and not giving the full immunity to the immunocompromised and elderly people,” Grandvaux says.
Choudhary points out that the delayed-second-dose strategy has advantages and disadvantages, and the ideal timing of the second dose is not established. But he urges people to use caution in between the first and second doses. “I will say it this way: when the population is vaccinated with the first dose, they need to be very careful. They shouldn’t be of the opinion that now they are immune and their immune system is robust,” he says.
***
In the fall of 2020, Abdool Karim was supposed to have been in Canada with his wife, fellow epidemiologist Quarraisha Abdool Karim. They were being honoured with the John Dirks Canada Gairdner Global Health Award for their discovery that antiretroviral medications prevent sexual transmission of HIV. This finding laid the foundations for the development of pre-exposure prophylaxis, also called PrEP, the prevention strategy that has changed the trajectory of HIV around the world. Instead of visiting Canada, the pair stayed home and recorded a video in which they each presented the award to the other.
From his office, with its framed photos of Nelson Mandela on the wall behind him, Abdool Karim recounts recent conversations with former colleagues who now live in Canada. They told him that some places in Canada had run out of ICU beds and were shipping patients to other hospitals. “But Canada is quite blessed in having a lot of vaccines available,” he says. “Other countries, like our own, have struggled to get enough doses.”
South Africa never had the option to view this period as a race between variants and the vaccine, he explains. By May 17, less than one per cent of South Africa’s population had received a dose of vaccine, compared to 45 per cent of Canadians. And yet, through the winter and spring, South Africa had far fewer cases of COVID-19—9,700 people were diagnosed with COVID-19 in the first week of May, about one-fifth of those in Canada. Transmission dropped to a relatively low level after restrictions were brought in in January, although cases began rising mid-spring.
The whole world needs vaccines, and quickly, to bring COVID-19 under control and reduce the risk of more variants emerging, Abdool Karim says. Studies so far suggest that vaccines hold up well against the variants of concern, with some reduced success against B.1.351 in South Africa. It’s too early to say whether future variants will be able to escape vaccine immunity.
Wealthy countries are controlling the vaccine supply—Canada among them. According to the Our World in Data project at the University of Oxford, only 0.3 per cent of the world’s vaccine doses have been given in low-income countries as of the third week in May. “And your country is a prime culprit in that regard because you bought about 10 doses of vaccine for each of your citizens,” says Abdool Karim. “We don’t know what you’re going to do with all those vaccines. So the rest of us have to join the queue.”
In the meantime, the virus keeps replicating.
Article From: Maclean’s
Author: Christina Frangou

